Potential new treatment options for
HER2-low and HER2-negative breast cancer patients
Summary
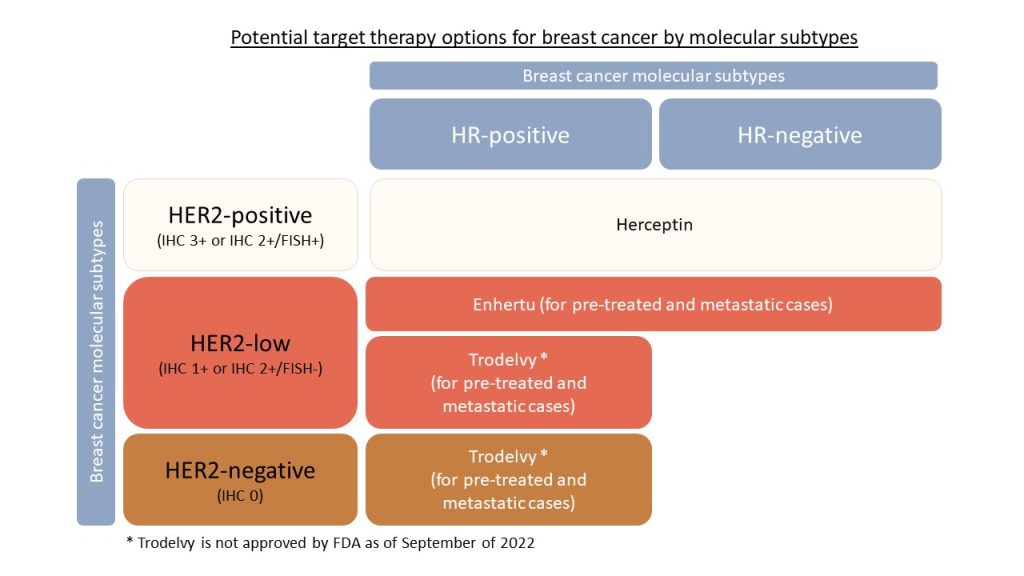
More options for HER2-low and HER2-negative patients
Two drugs, Enhertu and Trodelvy, should provide more target therapy options for breast cancer patients with HER2-negative and HER2-low diagnoses. In general, treatment options for HER2-negative patients with positive estrogen and progesterone (HR-positive) may be limited to hormone therapy.
Herceptin for HER2-positive
- FDA approved Herceptin, which dramatically improved prognoses for HER2-positive breast cancer patients, in 1998.
- Herceptin was the last breast cancer drug to get a standing ovation when Dr. Dennis Slamon presented the clinical trial results at the ASCO, American Society of Clinical Oncology, meeting in 1998. Please ask your doctor about the molecular subtype of breast cancer If you are unfamiliar with HER2-positive and HER2-negative subtypes of breast cancer.
HER2-positive subtype
- However, the threshold of meeting HER2-positive is high, and the IHC (Immunohistochemistry) test score needs to be 3+. Please ask your doctor about the IHC test score If you are unfamiliar with it. Breast cancer diagnostic of the IHC score of 0 or 1+ has been diagnosed as HER2-negative breast cancer until recently.
- Additional classification is required if your IHC score is on the borderline of 2+. The test is called a FISH test. When a FISH test result is positive, the subtype is HER2-positive when the IHC score is 2+. On the other hand, if a FISH test is negative, the resulting subtype is HER2-negative.
HER2-low subtype
- Now there is a new subtype, HER2-low breast cancer. It is the HER2-low subtype if
- the IHC score is 1+, or
- the IHC score is 2, and the FISH is negative.
- There was a 40-second standing ovation when Dr. Shanu Modi of Memorial Sloan Kettering Cancer Center presented this new concept of the HER2-low and the clinical trial results of Enhertu at the 2022 ASCO annual meeting.
Good news for HER2-low patients (Enhertu)
- Dr. Modi presented that a target cancer drug, Enhertu, improves endpoints for pre-treated HER2-low and HR-positive/HR-negative metastatic breast cancer patients.
- This presentation has been one of the most wonderful breast cancer treatment news since the news of Herceptin in 1998. FDA approved Enhertu in August of 2022.
- Therefore, there has been good progress in the breast cancer community for HER2-positive breast cancer and pre-treated HER2-low metastatic breast cancer. Is there good news for HER2-negative breast cancer? Yes, there is.
Good news for HER2-low or HER2-negative /HR-positive patients (Trodelvy)
- The good news is that Trodelvy (sacituzumab) significantly improves treatment outcomes in pre-treated HER2-negative(including HER2-low)/HR-positive metastatic breast cancer patients in an approved clinical trial.
- Please ask your doctor if your case fits in one of the classifications.
What is Trodelvy (sacituzumab) ?
Targeted therapy drug
- Trodelvy is a type of drug called Sacituzumab to treat cancer sold under the brand name Trodelvy.
- Trodelvy is a targeted therapy drug to fight cancer.
- It targets tumor cells while sparing healthy cells to treat those diagnosed with breast cancer.
Chemically combined drug
- Trodelvy is an antibody-drug conjugate, ADC, and is a conjugated drug in that two different substances join chemically, where the two substances are a proprietary hydrolyzable linker attached to SN-38, an active anticancer drug.
- Furthermore, It is a Trop-2-directed antibody-drug conjugate where Trop-2 is a cell surface antigen common in breast cancer.
Is Trodelvy (sacituzumab) an approved treatment? Yes!
Good results with HER2-negative/HR-positive patients
- The producer of Trodelvy, Gilead Sciences, Inc., announced in August 2022 promising results from their global clinical trials with certain patients treated with Trodelvy.
- The patients in the study are those diagnosed with HER2-negative/HR-positive metastatic breast cancer who received prior endocrine therapy, CDK4/6 inhibitors, and two to four lines of chemotherapy.
HER2-negative/HR-positive
- As a reference source, the HER2-negative/HR-positive breast cancer molecular subtype is a human epidermal growth factor receptor 2(HER2)-negative/hormone receptor(HR)-positive breast cancer.
- This subtype is the most common molecular subtype of breast cancer.
- Almost one in three cases of early-stage breast cancer eventually becomes metastatic, meaning cancer would spread to an area farther from where it started to another part of the body.
- HER2-negative/HR-positive metastatic cancer patients have a five-year relative survival rate of around 30%.
TROPiCS-02 clinical trial phase 3
- The global clinical trial is called the TROPiCS-02, a global, multicenter, open-label, and Phase 3 study.
- For reference, there are four phases in clinical trials, and the U.S. Food and Drug Administration (FDA) reviews and approves data from each phase.
- After successful Phase 3 trials, the FDA, looking at data from the three phases, determines if a drug developer should release it to consumers.
Good phase 3 results submitted to FDA
- Gilead announced statistically significant and clinically meaningful results from its second interim analysis.
- The results from the study’s key secondary endpoint, overall survival(OS). OS is a standard and promising clinical endpoint. Therefore, this is clinically meaningful.
- Gilead has submitted the Phase 3 data to FDA.
Including HER2-low cases
- The TROPiCS-02 study included patients with HER2-negative as well as HER2-low.
- As FDA approved the application, patients with HER2-negative/HR-positive and HER2-low/HR-positive metastatic breast cancer with the pre-treatment may have more treatment options.
- HER2-low is a newly defined subset of HER2-negative breast cancer.
Great news in February 2023!
- FDA approves Trodelvy for patients with HER2-negative/HR-positive and HER2-low/HR-positive metastatic breast cancer with the pre-treatment!
- Please see the details from the site.
What is Enhertu (trastuzumab deruxtecan (T-DXd))?
New target drug Enhertu
- There is another promising drug called trastuzumab deruxtecan with a brand name Enhertu, which AstraZeneca and Daiichi Sankyo jointly developed and commercialized for breast cancer treatment.
- Like Trodelvy, Enhertu is another type of antibody-drug conjugate (ADC), a precisely engineered HER2-directed ADC.
What are the Enhertu clinical trial results?
Good news for HER2-low patients
- There was a clinical trial called the DESTINY-Breast04 Phase III trial.
- The test has shown positive results for certain types of breast cancer patients.
- The types of patients are those diagnosed with HER2-low unresectable, meaning unable to be surgically removed, or metastatic breast cancer with HR-positive or HR-negative.
- Therefore, this is another good result for those patients with HER2-low breast cancer.
Better results with Enhertu with PFS
- Enhertu test results provided good endpoints with progression-free survival (PFS) and overall survival (OS) endpoints.
- The results were better than those of the standard of care physician’s choice of chemotherapy.
Progress-free survival (PFS)
PFS endpoint is another important endpoint for measuring the effectiveness of cancer drugs besides OS, and PFS measures how long a person lives without the cancer worsening.
49% reduction in risk
- The endpoint analysis of the study demonstrated a 49% reduction in the risk of disease progression or death.
- This result compared the physician’s choice of chemotherapy for HER2-low with HR-positive.
Better reported quality of life of patients
- On Sept. 11, 2022, Dr. Naoto Ueno presented the better patient-reported outcome (PRO) of DESTINY-BREAST-04 at the ESMO (European Society for Medical Oncology) in Paris.
- PRO reflects the patients’ perspective of the efficacy and safety of the drug treatment.
- For more detailed outcomes of the DESTINY-Breast04, please refer to the MD Anderson Cancer Center article and Dr. Ueno’s Facebook page.
FDA approval
The current availability of the approved Enhertu and Trodelvy in the US can lead to a new standard of care for cancer treatment patients in the US categorized as having HER2-negative and HER2-low breast cancer.
Conclusion
Near future availability of the approved Enhertu and Trodelvy can lead to a new standard of care for cancer treatment patients categorized as having HER2-negative and HER2-low breast cancer.
*Please see and ask your doctor about your cases. This article was created from various reference sources as a gate to multiple helpful pieces of information. Please refer to the original articles for more detailed information.
Source:
Trodelvy® (sacituzumab) Significantly Improves Overall Survival in Pre-Treated HR+/HER2- Metastatic Breast Cancer Patients in the TROPiCS-02 Study. Potential approval? Enhertu vs Trodevy. Which one first for HR+ low HER2? https://t.co/CMv1YXBHvf
— Naoto T Ueno, MD, PhD (@teamoncology) August 15, 2022
- Gilead Trodelvy press release
- AstraZeneca Enhertu press release
- HER2-positive breast cancer
- HER2-low breast cancer
We have provided these links to other websites because they may have information that would be of interest to you. There may be other websites that are more appropriate for your purpose. We do not necessarily endorse the views expressed or concur with the facts presented on these outside sites. Further, we do not endorse any commercial products that may be mentioned on these sites.
